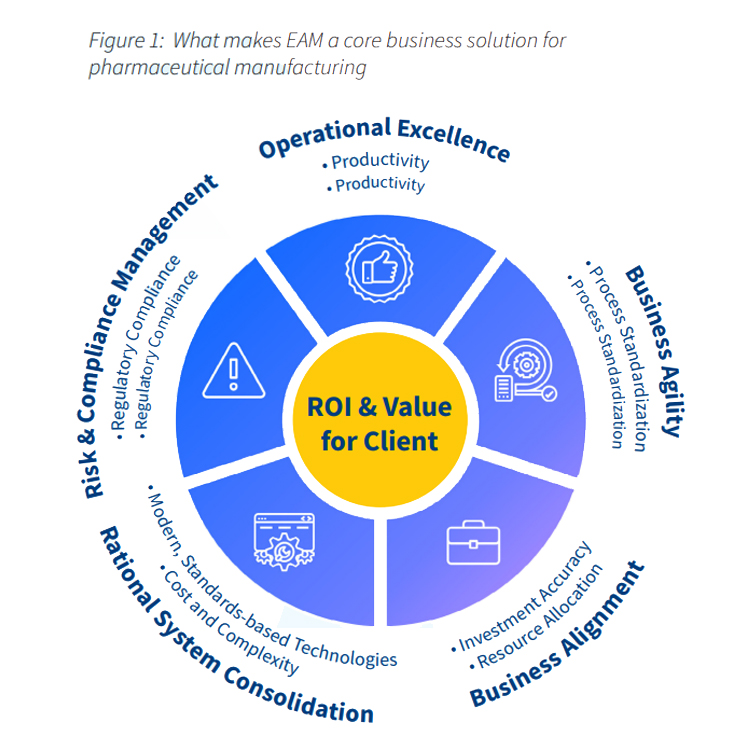
How the IBM Maximo® Asset Management solution benefits businesses in the Life Sciences sector
IBM’s Maximo® Asset Management solution is the market leader in enterprise asset management software. It is used by 14 of the 15 leading pharmaceutical organisations worldwide.
IBM Maximo® has enabled these companies to overcome the challenges of a heavily regulated life sciences sector, and minimise risk in their daily operations.
Companies using IBM Maximo® manage their assets effectively whilst maintaining a comprehensive, digital audit trail, and increasing the rate of first-time fix to key equipment and machinery.
An enterprise-wide asset management system helps life sciences companies to:
- Ensure compliance
- Reduce risk (and compliance costs)
- Maximize asset utilization & performance
- Boost product quality
- Reduce overall IT complexity
Compliance risk is reduced through the reduction of human errors, including decreasing paperwork and manual systems, removing duplication of work, and the availability of retrievable audit trails. Peacock Engineering can help you achieve all of this.
Ensure robust regulatory compliance
As life sciences businesses work hard to ensure compliance with the FDA’s Title 21 CFR Part 11 regulation, it is increasingly critical that they reduce compliance risk through preventive maintenance and instrument calibration activities. All of this is automatically documented in the IBM Maximo® system.
IBM Maximo® EAM software helps life sciences companies to:
- Improve regulatory compliance
- Maintain a comprehensive audit trail
- Increase operational efficiency
- Increase operational efficiency
- Increase uptime & first-time fix
- Extend the lifecycle of their plant & other assets
- Calibrate machinery
- Work remotely
- Identify individuals who need training
IBM Maximo® also allows you to identify individuals who might need better training, because the programme has exception reporting built into it. Peacock Engineering can help you to harness these features effectively in your organisation.
Make validation easier and cheaper
The life sciences sector is heavily regulated. Companies are under intense pressure to comply with Title 21 CFR Part 11, and demonstrate that new software is designed, developed, tested, deployed and maintained in accordance with the regulations.
We work with clients who use Maximo® as part of their Quality Management System (QMS), which can help to make validation more manageable.
In addition, many organisations struggle to describe their user requirements due to their lack of product specific knowledge. Because Peacock Engineering have a huge body of expertise in configuring Maximo, we can help you to precisely define your requirements, which helps to make the validation process clearer and simpler.
We then ensure that all aspects of the software and implementation are documented in detail. The combination of precise user requirements, and thoroughly-documented development, makes validation considerably easier to achieve, and reduces validation costs.
We also have a flexible approach to development, using a combination of agile and traditional waterfall techniques.
This enables us to tailor the development methodology to suit your needs, which results in:
- clearer documentation
- faster implementation
- lower validation costs
We helped a leading Life Sciences business to improve their validation, using IBM Maximo® delivered via our managed cloud service. Read the case study here.


Full digital audit trail
Using IBM Maximo® in your organisation will help to ensure that you have:
- A full audit trail
- Completely accurate data
- E-signatures for every work order
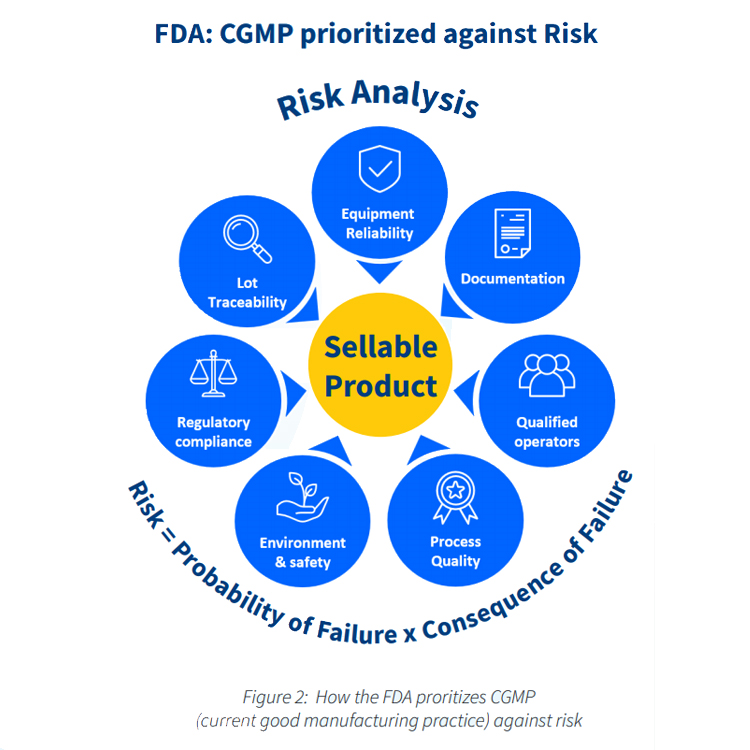
IBM Maximo® ensures that your pharmaceuticals are being manufactured to the highest CGMP standards, and meet all FDA and other requirements. The embedded audit trail helps you to prove that all your processes have been carried out correctly, at every stage.
It also means that, if you need to recall a product, you know the boundaries of which products need to be recalled and which don’t.
You can narrow the issue down to a particular batch, product type, or date, and resolve any manufacturing issue faster. This will help to avoid unnecessary product recalls, reducing your costs.
Reduce risk in your daily operations
IBM Maximo® helps life sciences companies to reduce the incidence of breakdowns and accidents to near zero. It guarantees that all equipment is proactively looked after by constantly monitoring key indicators.
This proactivity is what gives IBM Maximo® a major advantage over other asset management systems. It ensures that you:
- Take a proactive intervention approach
- Use data to identify equipment problems
- Adhere to all health & safety standards
- Keep your staff secure at all times
Demonstrate fulfilled work processes
Life sciences companies are under intense pressure from industry regulators to demonstrate that all their processes are designed, developed, tested, deployed and maintained to the required standards. Utilising the inherent design flexibility of IBM Maximo® allows it to be configured to comply with business process needs and regulatory requirements.
Furthermore, because the system requires a digital signature for every work order, this means that:
- An audit trail always exists
- Regulators can verify that all steps were taken
- Your staff are completely safe at all times
Increase operational efficiency
The IBM Maximo® solution ensures that:
- You are compliant
- You have an infallible digital audit trail
- Quality is continually maintained
IBM Maximo® significantly reduces the scope for human error, which improves efficiency and reduces costs. And because IBM Maximo® is a totally digital system, slow, paper-based processes can be eliminated, further improving efficiency.


Increase asset uptime and first-time fix
IBM Maximo® flags up issues instantly when a problem occurs with your equipment. This means that work orders are sent out straight away, and the problem is dealt with as soon as possible.
Without this system, scheduled checks may result in a faulty asset remaining in use for a longer period of time. IBM Maximo® immediately highlights the specific asset that has the problem, and what the problem is.
This means that:
- the right technician is called out
- the problem is correctly dealt with
- improved first-time fix rate
- increased uptime for the asset
Extend the lifecycle of your assets
Extending the life of your plant, and other assets, is a challenge for life sciences businesses. IBM Maximo® makes this easier by escalating problems with your assets quickly, so that you know exactly what the problem is, what is needed to resolve it, and how and when it will be fixed.
This ensures that the right technician is called out to fix it, and that they have the right level of expertise according to the complexity of the problem at hand. As a result, your asset will be fixed correctly and by the right person, which reduces the risk of a fault re-occurring, and therefore extends the life cycle of your asset.
We helped PCI Pharma to plan their maintenance more efficiently using IBM Maximo®. Read the case study here.
Work effectively from remote locations
Peacock Engineering’s Fingertip mobile solution for IBM Maximo® is used by many life sciences organisations. With its real-time link with Maximo, it is ideal for enforcing safety-critical processes, and generating work orders in the field, in response to the current situation.
Fingertip is also ideal for the life sciences sector, because it can be used in hazardous and volatile environments in suitable ATEX or DSEA-compliant devices. Fingertip can operate fully without an internet connection; as soon as the connection is restored, Fingertip updates Maximo® with its current data.
Fingertip allows engineers and technicians to maintain and update your assets at remote locations, or in volatile environments, which means that:
- Your system is always current
- Human error is significantly reduced
- No duplication of work, or manual systems
- Work orders generated remotely, for faster fix times
- Your critical processes are enforced
- You have a digital audit trail for all work carried out
Fingertip also has several add-ons which are particularly useful for life sciences businesses. One of these is our Mobile Forms add-on, which can help you reduce risk in your daily operations.


This can be configured to ensure that your processes are followed precisely at the point-of-work, with digital sign-off required at every stage.
Learn more about Fingertip Mobile Forms here.
Fingertip also allows calibration teams to carry out offline calibration – which is not available with any other mobile solution currently.
Our clients report improvements in productivity of up to 30% from implementing a mobile strategy combining Fingertip with IBM Maximo®.
Peacock Engineering helped Guerbet, a world leader in medical imaging, to extend IBM Maximo® into the field and record data from remote locations. Read the case study here.
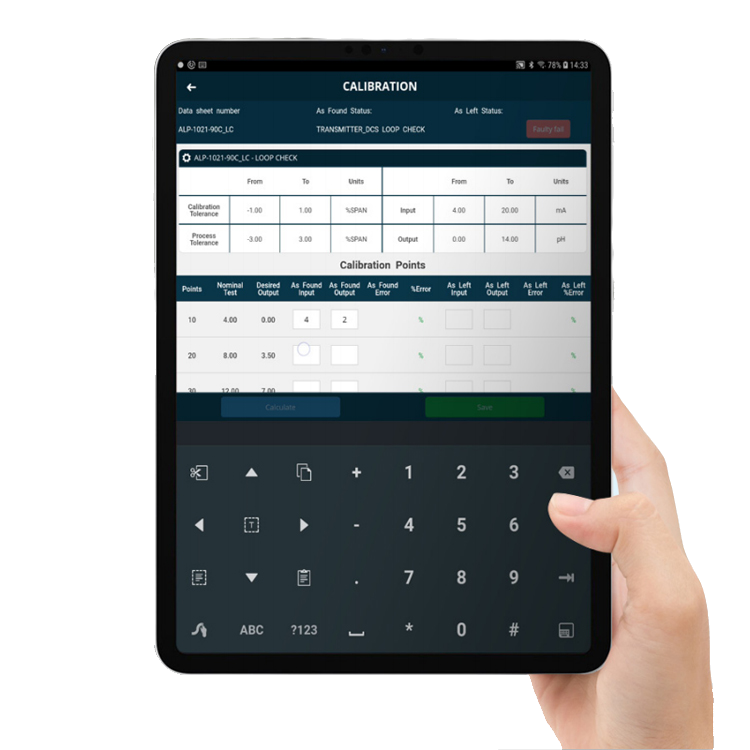
Calibrate your equipment and tools
IBM Maximo® and Fingertip can be extended to include various optional features, such as calibration, when required.
Fingertip’s Calibration Module enables calibration at the point-of-work. This is particularly useful for life sciences businesses, where precision calibration of equipment and tools is critical. Calibration at the point-of-work is also a real time saver, as it avoids having to take readings manually, return to the office to enter readings, go back to the equipment, take more readings, and so on.
Fingertip’s Calibration Module can help you to:
- Maintain regulatory compliance
- Improve data quality
- Ensure a full digital authorisation trail
- Significantly reduce time spent on the calibration process
Fingertip Calibration, and other modules, can be added at any time. Learn more about Fingertip Calibration Module by watching this video.